The current position:Home > Information dynamic
> Industry Trends
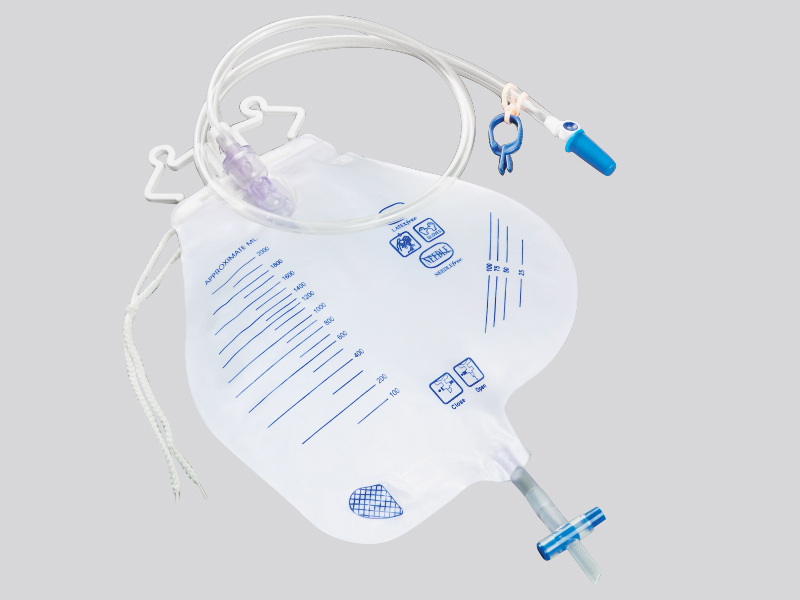
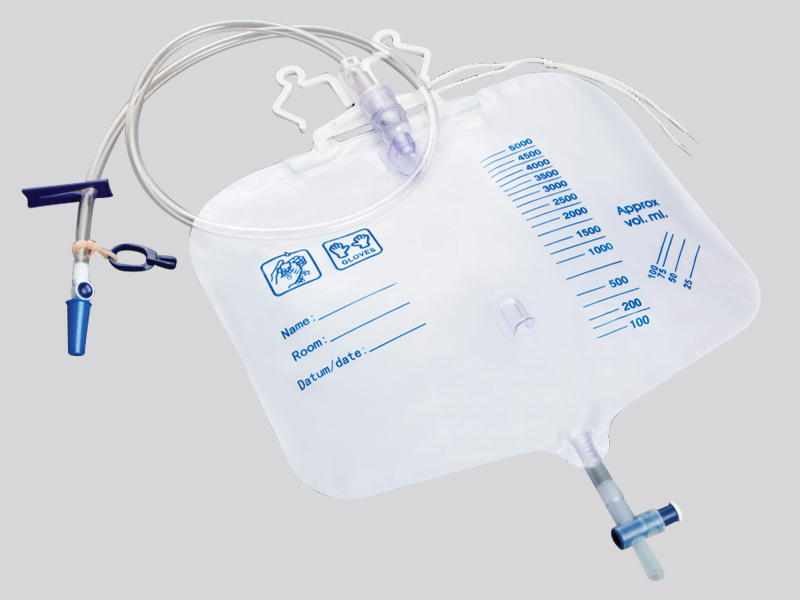
Where is the hygiene and safety of disposable feeding bags reflected
source:www.cnboyi.net | Release time:2025年08月04日1、 Safety and biocompatibility of raw materials
1. Selection of medical grade materials:
Feeding bags typically use high molecular weight materials that comply with medical device standards, such as polyethylene (PE), polypropylene (PP), or polyvinyl chloride (PVC), which must meet medical grade standards and be free of harmful plasticizers such as phthalates. These materials are chemically stable, non-toxic, odorless, and not easily react with food/nutrient solutions to prevent harmful substances from migrating into the contents.
2. Biocompatibility certification:
Raw materials need to undergo biocompatibility testing (such as cytotoxicity and allergenicity tests) to ensure that they will not cause adverse reactions such as allergies or inflammation when in contact with the human body (or indirectly with nutrient solutions), especially for sensitive populations such as infants and postoperative patients.
2、 Aseptic production and packaging technology
1. Clean environment production:
The entire production process of feeding bags is carried out in clean workshops of 100000 or higher levels to avoid contamination by microorganisms and dust in the air. Production equipment needs to be disinfected regularly, and operators need to wear sterile protective clothing to reduce the risk of human contamination.
2. Sterilization treatment:
Depending on the product type, methods such as ethylene oxide sterilization and radiation sterilization (such as gamma rays) may be used to ensure that the finished product meets the sterile requirements (in accordance with the "Hygiene Standards for Disposable Aseptic Medical Devices"), especially suitable for enteral nutrition feeding scenarios that directly enter the digestive tract.
3. Sealed packaging protection:
The finished product is packaged in sterile sealed packaging (such as aluminum-plastic composite film), and the packaging material has good barrier properties, which can prevent contamination by external microorganisms, moisture or pollutants during transportation and storage. When the packaging is damaged, it can be directly identified by appearance, avoiding the use of contaminated products.
3、 Anti pollution design and safety in use
1. Unidirectional valve and closed structure:
Feeding bags are usually equipped with one-way valves, sealed interfaces (such as Ruhr interfaces), and other designs to ensure that the nutrient solution is not easily leaked after injection, while preventing air, dust, or reflux liquid from entering the bag and causing contamination. Some products also come with interfaces to reduce the risk of cross contamination during operation.
2. Disposable characteristics:
Compared with reusable containers, disposable feeding bags avoid residual contamination caused by poor cleaning (such as food residue breeding bacteria and cleaning agent residue), fundamentally eliminating the risk of cross infection that may arise from repeated use, especially suitable for immunocompromised patients (such as critically ill patients and newborns).
3. Easy to tear and clearly labeled:
The opening of the packaging is designed to be easy to tear and will not contaminate the bag during the tearing process; The bag body is usually marked with information such as production date, expiration date, sterilization method, and scope of application, which facilitates users to confirm whether the product is within the safe cycle and avoid using expired or unqualified products.
4、 Quality Control and Compliance
1. Strict factory inspection:
Each batch of products needs to undergo visual inspection (no damage, no foreign objects), sealing testing (no leakage under pressure), sterile sampling, etc., to ensure that products that do not meet hygiene standards will not enter the market.
2. Compliant with industry standards:
It is necessary to comply with international medical device standards (such as China's GB 15980-1995 "Hygienic Standards for Disposable Medical Supplies" and ISO 10993 series biocompatibility standards), and obtain approval from regulatory authorities (such as medical device registration certificates) to ensure its health and safety bottom line from a regulatory perspective.
5、 Adaptability and ease of use reduce operational pollution
1. Compatible with feeding equipment:
The interface design of the feeding bag is matched with enteral nutrition pumps, syringes, and other instruments, with a simple connection process and strong sealing, reducing liquid overflow or air entry during operation and lowering the probability of contamination.
2. Reduce steps for one-time operation:
Without the need for pre-treatment steps such as cleaning and disinfection, it can be used directly after unpacking, shortening the operation process and reducing the risk of contamination caused by human contact. Especially in emergency medical scenarios, it can better ensure hygiene and safety.
Prev:
What industries are disposable enema bags used in
Next:
What are the advantages of disposable negative pr…

 Cn
Cn En
En WeChat ID:
WeChat ID:





 Contact us
Contact us
 Add WeChat
Add WeChat
 Telephone
Telephone